GxP SUITE
21 CFR part 11 compliant imaging toolbox
The 21 CFR part 11 / EU GMP Annex 11 regulations establish criteria for electronic records and electronic signatures to be trustworthy and reliable.
The goal is to minimize the chances of data falsification and maximize the chances of detecting falsification.
To achieve this, we have created the GxP SUITE for your imaging projects - all specifically designed for SYNENTECs imaging and automation solutions.
Discover all the advantages
plug and play solution
GxP SUITE
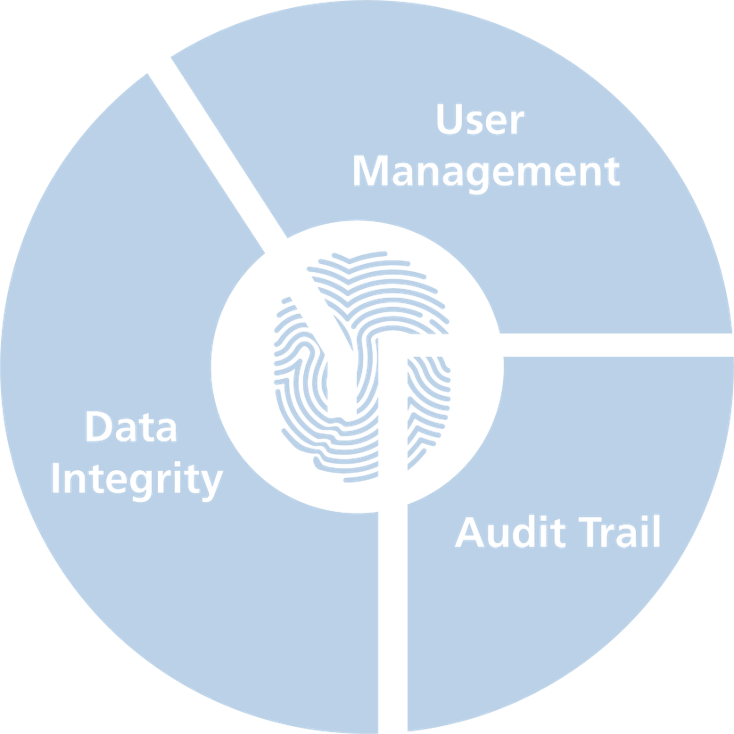
Our three mainstays of 21 CFR part 11 compliance
User Management
- Group items
- Security management by customer‘s IT
- YTUser / YTManager / YTAuditor
- Certification by training
Data Integrity
- Local database
- Digital fingerprinting technology
- Traceable validated experiment format
- Data integrity check
Audit Trail
- Full traceability
- Multiple entries searchable and filterable
- Exportable to pdf
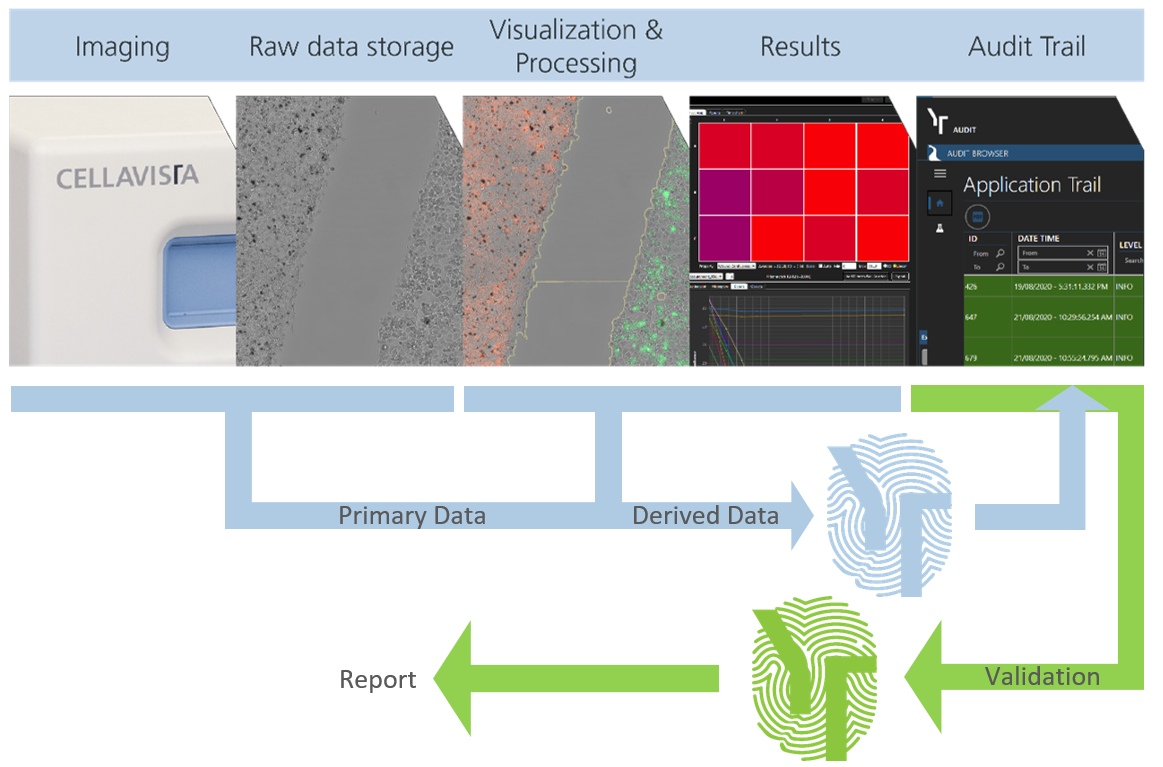
Data integrity made simple
Workflow of advanced safety features
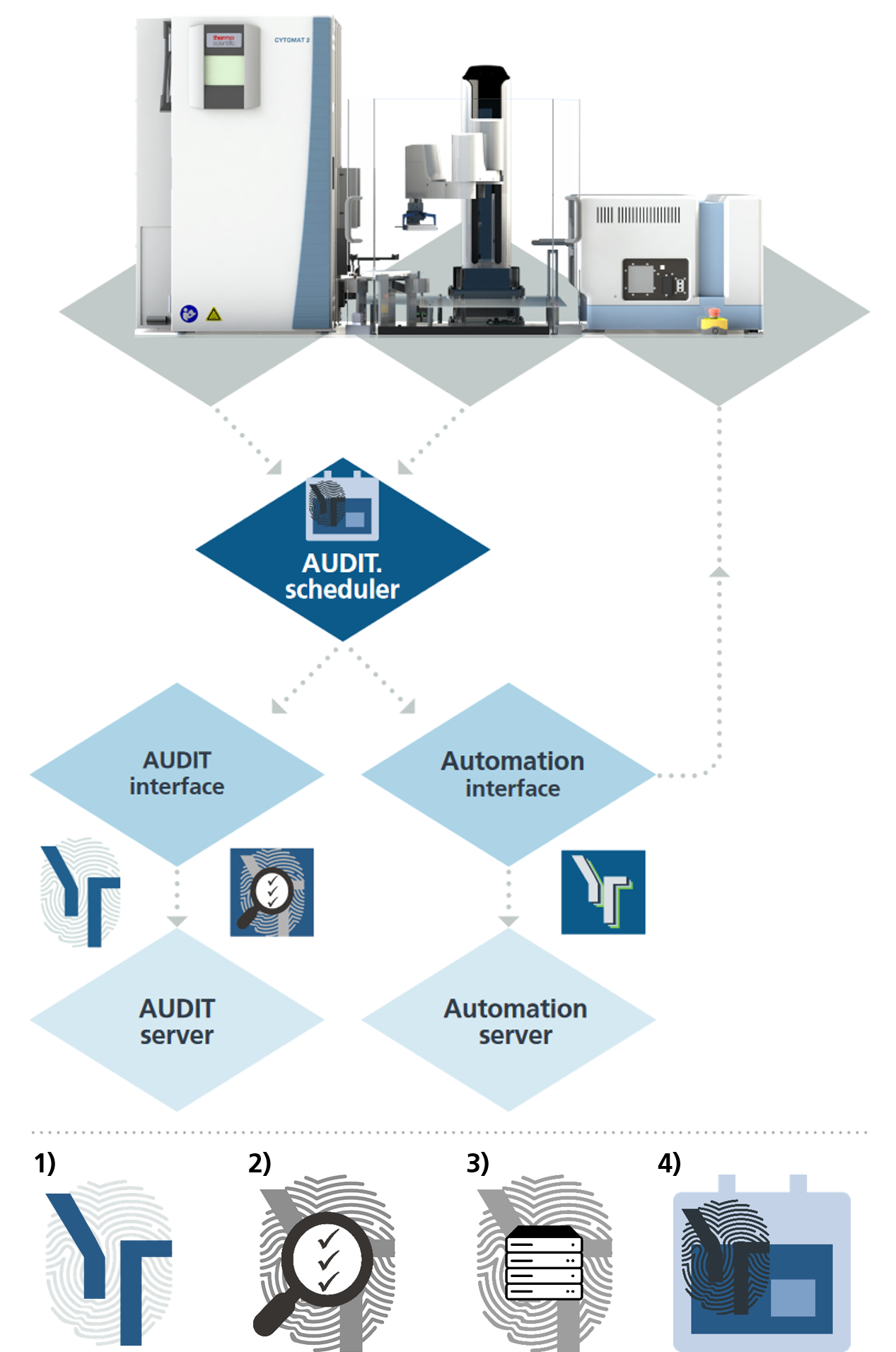
21 CFR part 11 compliant imaging and data handling
Validated automation set-up
This automation scheme shows SYBOT-1000 automation with CELLAVISTA 4K High End and Cytomat 2-C LiN. All processes including the Batch Processing are audited completely and result in a 21 CFR part 11 compliant imaging processes. Using AUDIT Server enables fully flexible usage of the imaging systems even providing a continuous audit trail even when an experiment is imaged on different imagers at different points in time. This feature can also be applied to third party automation workcells involving multiple imaging systems.
Data integrity made simple
Validated environment from a single source
Download BrochureSYNENTECs GxP SUITE uses advanced digital fingerprint technology and audit trailing in a highly automated manner for maximum 21 CFR part 11 compliant high-throughput cell imaging - all specifically designed for SYNENTECs imaging and automation solutions.
DO YOU WANT TO KNOW MORE?
We know that time is an increasingly scarce resource, even in the lab. That's why we've thought your problem through and have everything ready for a complete one-handed solution.



